Abstract
Introduction. Despite the increasing of number of patients with Sickle Cell Disease (SCD) in Italy, due to multi-ethnic migratory phenomena, a large percentage of Caucasian sickle population is already present in Italy mainly with b-thal/HbS genotype. Red cell transfusion is one effective treatment for both acute and chronic complications of SCD, while hydroxycarbamide (HC) is used to reduce the frequency of painful vaso-occlusive crises (VOCs) and decrease the need for blood transfusion.
Through the National Comprehensive Reference Centers for SCD, the Italian Society of Thalassemia and Hemoglobinopathies (SITE), in collaboration with the Society Italian Transfusion Medicine and Immunohematology (SIMTI) and the Italian Association of Hematology and Pediatric Oncology (AIEOP) conducted a national survey to collect information on different therapeutic approaches used for SCD patients.
Aim. To assess therapeutic approaches used a large Italian cohort of patients with SCD, accounting for age, genotype and ethnicity.
Patients and Methods. Observational Longitudinal Systemic Multicentre Study (https://clinicaltrials.gov/ct2/show/NCT03397017). Data were collected from 2015 to 2018 through a standard web-based application (www.SITE-italia.org) encrypted by the Central Server. All the SCD patients, treated or not treated, were included in order to identify the overall number and all gave written informed consent. The study was approved by Ethics Committee of Fondazione IRCCS Ca' Granda, Ospedale Maggiore Policlinico of Milan, Italy.
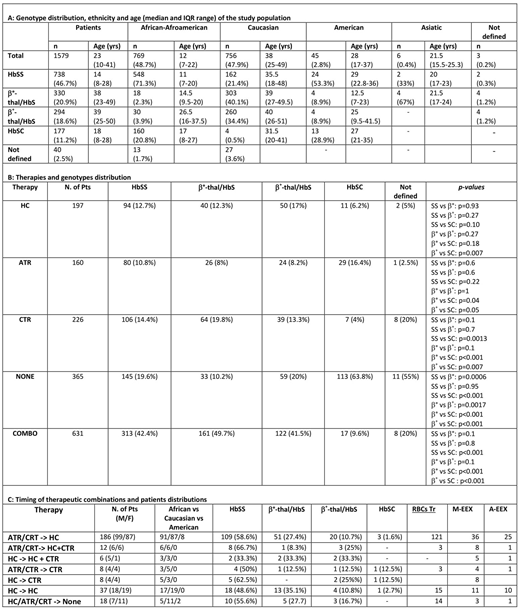
Results. Thirty-four centers were involved from 14 Italian regions and 1,579 patients were enrolled (802 male and 777 female; median age 23 years - IQR, 25th-75th 10-41 yrs). Genotype, age and ethnicity distribution are shown in Table 1A. As expected, the median age of non-Caucasian patients, mainly HbSS, is significantly lower than Caucasian ones (p<0.001).
Out of 1,579, 365 SCD patients (23%) did not receive any therapy. Acute transfusion regimen (ATR), Chronic transfusion regimen (CTR) and HC were given in monotherapy, respectively in 160, 226 and 197 patients, or in succession/combination in 631 (Table 1B), distributed throughout genotypes. The main reasons for ATR were acute anemia (384 events) and VOCs (352), followed by acute chest syndrome (ACS; 170), surgery (82), pregnancy (64), splenic sequestration (26), stroke (9); multi-organ failure (MOFs 6) and priapism (5). For CRT, it was acute anemia (306 events) and prevention of VOCs (371), ACS (107), primary stroke prevention (78) and secondary prevention stroke (55), pain HC-resistent (39) and leg ulcers (12). For 275 patients out of 631 it was possible to follow the timing of therapy switching (Table 1C). Of 275 patients, 67.6% switched from ATR/CRT to HC, 2.9% from HC to CRT and 6.5% stopped every therapy. Out of 275 patients, 104 were treated with overlapping therapeutic regimen.
Discussion. The significant difference of age in Caucasian and non-Caucasian patients is probably due to the efficacy of the national prevention program of hemoglobinopathies, because the non-Caucasian patients are prevalently born out of Italy.
The transfusional approach is similar in HbSS and b°-thal/HbS and b+-thal/HbS patients regarding both ATR and CTR. HbSC genotype needed less therapies(p <0.001), confirming a less severe clinical pattern. About the combo or sequential therapy, HC was the more frequent chronic therapy used lifelong, mainly in patients with HbSS, because of wide spread of age and transfusional match problems due to different ethnicity.
Summary/Conclusion. The transfusional approach is similar in HbSS, b°-thal/HbS and b+-thal/HbS patients with similar indications, prevalently VOCs and anemia. The significant higher age in Caucasian cohort and the consequent long term follow up could be the cause of variable therapeutic approach observed, however Hydroxycarbamide seemed to be the therapy more frequently used and finally suggested to manage chronic manifestations.
Origa:Apopharma: Honoraria; Novartis: Honoraria; Bluebird Bio: Consultancy; Cerus Corporation: Research Funding. Forni:Apopharma: Other: DSM Board; Celgene: Research Funding; Novartis: Other: travel expenses, Research Funding; Shire: Research Funding; Roche: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal